When accounting for IVF, genetic testing, donor services, and reproductive tissue storage, the fertility market in the US is estimated to be more than $8B One in five individuals struggle to conceive, and yet only 2% of newborns are conceived through IVF. The main reasons why IVF isn’t used more frequently are the astronomical costs, the intensive and often isolating effort required, and the associated discomfort. Gameto is a biotech company that is focused on women’s reproductive health using cell engineering. The company’s first commercial candidate, Fertilo, works like “ovaries in a dish” and is designed to reduce the need for injections and also truncates the timeline for IVF and egg-freezing to 2-3 days from the customary 10-14 days. Gameto also has a program available for sperm fertility and is looking at new ways to expand its cell engineering to tackle conditions associated with menopause. Fertilo is currently being used in Australia and Latin America and the company is working to support the commercial launches in the near term. Gameto’s work represents a promising step forward in reproductive medicine, offering hope to individuals struggling with fertility issues.
AlleyWatch caught up with Gameto CEO and Cofounder Dr. Dina Radenkovic to learn more about the inspiration for the business, the company’s strategic plans, latest round of funding, and much, much more…
Who were your investors and how much did you raise?
Gameto raised a Series B round of $33M, bringing the total funds raised to $73M to date. Two Sigma Ventures and RA Capital Management led the Series B with participation from existing investors, including Insight Partners, Future Ventures, and BOLD Capital Partners. New investors included Olivia Walton’s Ingeborg Investments, AmplifyHER Ventures, Stacey Bendet Eisner, Founder, CEO, and Creative Director of alice + olivia, and Chelsea Hirschhorn, Founder and CEO of Frida.
Gameto is also supported by existing investors including Anne Wojcicki of 23andMe, Bob Nelsen of ARCH Venture Partners, Arcadia Investment Partners, Overwater Ventures, Plum Alley, Lux Capital, FJ Labs, SALT Fund, Myelin VC, TA Ventures, Jack Abraham of Atomic, YES VC and Dan Rose.
Tell us about the product or service that Gameto offers.
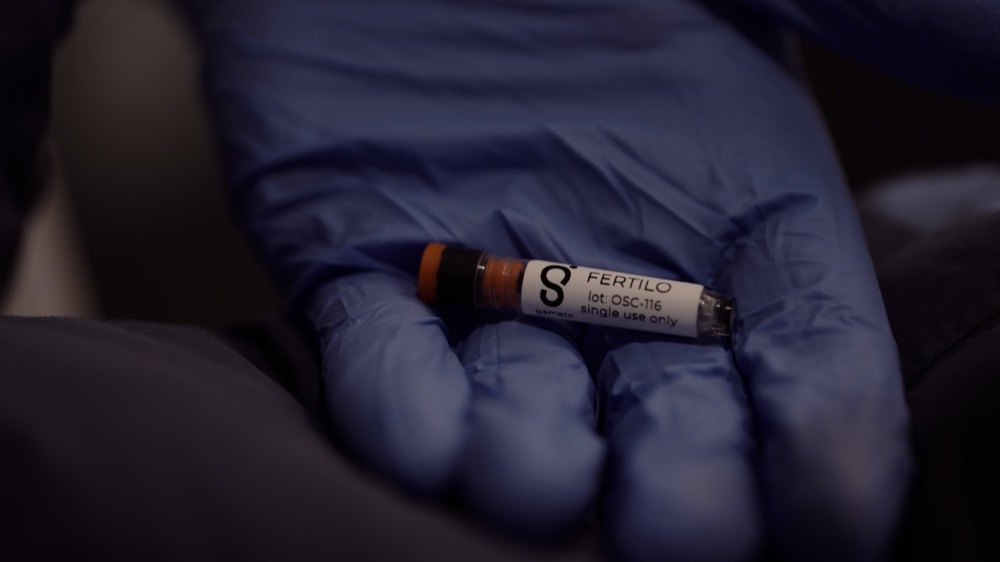
Gameto is a biotech company that uses cell engineering to develop therapies to improve the female reproductive journey. Our platform and technology allow us to model ovarian biology and conduct rapid testing of novel treatment candidates. Through our work with the Wyss Institute at Harvard, we used cell engineering to develop “ovaries in a dish,” which eventually led us to our lead program, Fertilo.
Fertilo is designed to make the IVF and egg-freezing processes faster, safer, more comfortable, and more accessible. Specifically, Fertilo is a novel investigational in vitro maturation (IVM) solution containing engineered ovarian support cells to mature eggs outside of the body. Our approach uses cellular engineering to create ovarian support cells from donor stem cells that recreate the natural environment in the ovary where egg cells would normally mature. This technology is designed to replace hormonal injections and shorten the in vitro fertilization (IVF) and egg-freezing cycle from 10-14 days to 2-3 days.
What inspired the start of Gameto?
 As a physician and researcher, I actually ended up in women’s health because I was working in the disease prevention and aging space, and that’s where I started to work on ovarian aging, which happens more quickly than other organs in the human body. This leads to infertility, menopause and other diseases of the female reproductive system. Ultimately, our team and I want to improve and develop better treatments for women’s health, and that led us to create Gameto.
As a physician and researcher, I actually ended up in women’s health because I was working in the disease prevention and aging space, and that’s where I started to work on ovarian aging, which happens more quickly than other organs in the human body. This leads to infertility, menopause and other diseases of the female reproductive system. Ultimately, our team and I want to improve and develop better treatments for women’s health, and that led us to create Gameto.
We start from first principle that having good models for disease to rapidly test treatment candidates is the best way to accomplish this. For example, IVF hasn’t really changed much since its inception and it can be a big burden for women. Female patients’ pain, morbidity, and inconvenience are frequently minimized and neglected in the healthcare setting, and often more so for women pursuing fertility treatments and egg or embryo freezing. We want Gameto to be that company that fills the void in the biopharma space for women’s health, with the goal of providing not only safe and effective treatments, but really focusing on the female patient experience.
How is Gameto different?
We’re the first ones to apply cell engineering in women’s health. We’re really pioneering this technology because we’re one of the first to use a female allogeneic cell line for clinical and commercial use. Our approach essentially creates an active cell therapy in a dish, which differs significantly from the media solutions that are currently used for in vitro maturation.
Because this is such a novel product, there are also a lot of regulatory considerations at play. We’ve been working with regulatory agencies to figure out how best to get these products through the regulatory review process so that they can potentially be used commercially.
What market does Gameto target and how big is it?
Our technology has the potential to address a wide range of women’s health issues, from fertility to other diseases of the reproductive system. We are first targeting the fertility market, which is large at approximately 5 million global IVF cycles and growing at ~7% per year, but with significant unmet need The global fertility market is estimated to be worth approximately $35.2B, growing at ~15.5% through 2028. Today, roughly one in five people struggle with infertility, and yet only 2% of babies are born from IVF. The IVF process requires ~2 weeks of hormonal injections, making it challenging, time-consuming, expensive, and potentially dangerous, which prevents many would-be consumers from pursuing fertility treatments.
Societal, demographic, and health factors, such as delayed parenthood, increased same-sex couples, and increased rates of chronic diseases, are expediting the need for fertility treatments. One of our programs, Fertilo Sperm, specifically addresses male factor infertility, which accounts for 40 – 50% of infertility cases.
Gameto also plans to target other areas in women’s health, including the menopause market. Gameto’s platform could be used to reduce the medical effects by integrating into the chemical conversation in the body and providing the hormones needed in real time. Menopause represents a major market with over 1 billion women experiencing menopause by 2025. Symptoms are estimated to cost >$2,000 per person per year in healthcare utilization and lost productivity. The menopause market, including its symptoms, is estimated to be a $600B opportunity.
What’s your business model?
Fertilo would be sold to clinics, and it’s also procured by the clinics. The business model is similar to embryo testing or other fertility and media solutions, as well as the fertility treatments, diagnostics, and medical device industries.
How are you preparing for a potential economic slowdown?
We’re already focused on advancing a single asset and one that’s cleared for commercialization in several countries. Within the biotechnology industry, we’ve experienced an economic slowdown for the past few years, so I don’t think things will change significantly. Even in an economic downturn, people may delay bigger purchases, but they’re not going to delay having a baby if they really want to. We’ve seen interest in IVF be resilient and even grow despite inflation and economic slowdown.

What was the funding process like?
Despite a challenging market, we’re pleased to see increased investor confidence in our platform technology and a recognition of the pressing need for modern treatments in the historically underfunded women’s health space.
What are the biggest challenges that you faced while raising capital?
Gameto sits at the intersection of life sciences and consumer and we’re proud of the strong mix of our biotech and deep tech investors. Because of our unique position, one challenge is that investors and funds are not as educated about the women’s health space, and they have to devote a lot more time and resources to get up to speed. That’s simply because there are not many companies in this space, so when teams are competing in hot areas of biotech – like cell therapies for cancer, GLP-1s for obesity, or AI for drug development – it may be easier for funds to do diligence. When they’re evaluating Gameto, they have to learn about an entirely new field, and there haven’t been many companies in the women’s health space taking a biopharma approach. As a result, there are fewer comps to look at and fewer success stories, so you really have to prove every argument rather than raise capital based on previous successes. However, the investors we’ve spoken with have acknowledged the novelty of our approach, the clear need it fulfills and the robust data we have shown.
What factors about your business led your investors to write the check?
Our investors have recognized our strong scientific foundation, dedicated and high-performing team, innovative pipeline, and encouraging data that underlie our technology. We take a scientifically rigorous approach and have published our research extensively, received different regulatory classifications in foreign jurisdictions, and received clearance for commercialization in Australia and also in large markets in Latin America.
What are the milestones you plan to achieve in the next six months?
We are focused on a few scientific and commercial goals through the end of the year. we’re working to support the commercial launches of Fertilo in Australia and Latin America, where it’s already being used in the clinic. We also look forward to publishing more of our research and advancing our clinical development plans for Fertilo.
What advice can you offer companies in New York that do not have a fresh injection of capital in the bank?
Whatever your business is, focus on proving out one thing and doing it very well, keep the burn low, and never compromise on the quality of your team.
Where do you see the company going in the near term?
In the near term, we hope to see more patients benefit from Fertilo and we’re looking forward to generating more robust clinical datasets of our product. In parallel, we’re also getting ready for the first launches of Fertilo globally in areas where it has been cleared for commercialization.
What’s your favorite summer destination in and around the city?
Central Park! I love to go on short and long runs there.





